Fraser Macdonald: Conservatism is killing Canadians (but not in the way you think I mean)
Canadians should expect a thorough and methodical regulator when it comes to their health. But these are not ordinary times.
By: Fraser Macdonald
As the second wave of COVID-19 rolls across Canada, it’s time to take a critical look at the performance of our national institutions.
Health Canada has an outsized role to play in this type of national crisis, and its performance so far shows why good intentions and the fear of political reprisals can lead to harmful inaction.
Our federal health department is known globally by pharmaceutical and medical device companies for its conservative, risk-averse approach and sluggish pace, especially when it comes to reviewing and approving new drugs and technology.
There’s a historical reason for this — the legacy of their failure on Thalidomide. The U.S. Food and Drug Administration (FDA) performed its duties correctly and demanded independent evidence on the safety of the drug. They famously refused to approve Thalidomide, while Health Canada’s predecessor (the Food and Drug Directorate) did. For valid reasons, Health Canada has thus always been a slow but thorough regulator who ensures every piece of evidence is derived internally or vetted by their own scientists.
In ordinary circumstances, this is arguably just fine. Canadians should expect a thorough and methodical regulator when it comes to their health.
But these are not ordinary times. Health Canada’s failure to approve vital medical technology like point-of-care testing has hamstrung the provinces’ ability to respond to the pandemic, and will ultimately cost Canadian lives.
Under the FDA’s Emergency Use Authorization (EUA) procedures, the U.S. regulator has approved over 100 COVID tests from different manufacturers. This includes 58 different serology tests and seven different Antigen tests. Just this week, the FDA issued the first EUA for an at-home COVID test. Health Canada has approved just six tests that could be used in point-of-care, while almost 100 languish in the approvals pipeline.
The U.S. FDA's rationale in granting these EUAs is simple. They saw that quickly allowing a range of valid testing tools to be part of the response toolbox can only help the front-line personnel battling COVID. This is rational, risk-proportionate decision making, and it does not mean the agency has not been prudent. At least two EUAs for serology studies have been revoked as the agency monitors data and performance relating to these studies.
Here in Canada, as Ontarians spent their September waiting in line to get tested, some for entire days, the federal government told us that rapid testing would make COVID worse. They argued that the regulator, Health Canada, must not be interfered with by politicians.
Then, under pressure from the opposition, they announced that they had purchased millions of dollars worth of tests from one manufacturer prior to its approval — and the necessary approval magically appeared the next day. We’re now being asked to believe that the miraculous timing was just a coincidence, and political pressure had no bearing on this decision.
But there’s actually nothing wrong with this “political pressure.” It is the job of Parliament and the government of Canada to demand that government agencies do their jobs efficiently and effectively.
Right now, Health Canada is failing Canadians. While the FDA made a reasonable risk assessment that the upside of timely access to diagnostic tools via EUAs and continued monitoring was worth the risk, Health Canada continues to drag its feet.
Undoubtedly, Canada has a number of endemic factors working against us. We’re a smaller market than the U.S., and fewer companies spend the time and energy applying for Health Canada approval as a result. But this is a further argument for a simplified, faster approvals process — one that makes companies more likely to bring their innovation here.
When it comes to vaccines, we can’t afford to lag behind again. With this laggard pace in mind, it’s not hard to imagine a scenario where the rest of the developed world is being vaccinated while Canadians sit around waiting for our regulator to triple check the math.
The government deserves some credit for leading the world in advance purchasing of vaccine doses. But none of these can be distributed without Health Canada approval, and their performance on testing leaves us with serious questions about their ability to deliver approvals as fast as other global regulators.
While Canadians watch in horror as COVID ravages the U.S., we must not fall prey to the trap of believing that everything in Canada is automatically better as we often do when discussing health care. Yes, we’re taking more precautions on mask wearing and social distancing, but when it comes to vaccine distribution (and, almost certainly, approval once we get to that stage), we are miles behind.
The U.S. has a plan for vaccine distribution — Operation Warp Speed. It’s clear, it’s written down, it even has its own website. Here in Canada, the federal government won’t even tell us how many doses are going to each province.
When this is all over, there will undoubtedly be a royal commission that looks into the federal pandemic response and Health Canada’s role in it. We’ll have a report in 10 years’ time. But we should expect and demand improvement now, not in 10 years.
From both a health and economic standpoint, every day we delay the start of vaccination will mean lives lost and businesses closed. If ever there was a time to shift priorities and get results, this is it. And the Trudeau government has a responsibility to demand more of Health Canada — even if critics in the gallery cry “political interference.”
Fraser Macdonald is a lawyer and public affairs consultant based in Toronto, and a fellow at the Canadian Freedom Institute @CanFreedomInst
The Line is Canada’s last, best hope for irreverent commentary. We reject bullshit. We love lively writing. Please consider supporting us by subscribing. Follow us on Twitter @the_lineca. Fight with us on Facebook. Pitch us something: lineeditor@protonmail.com

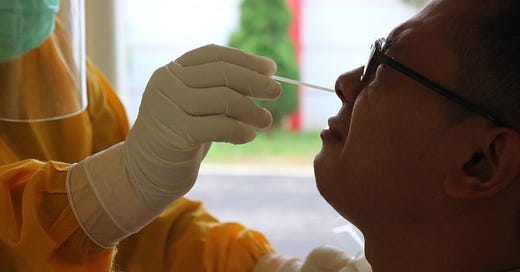


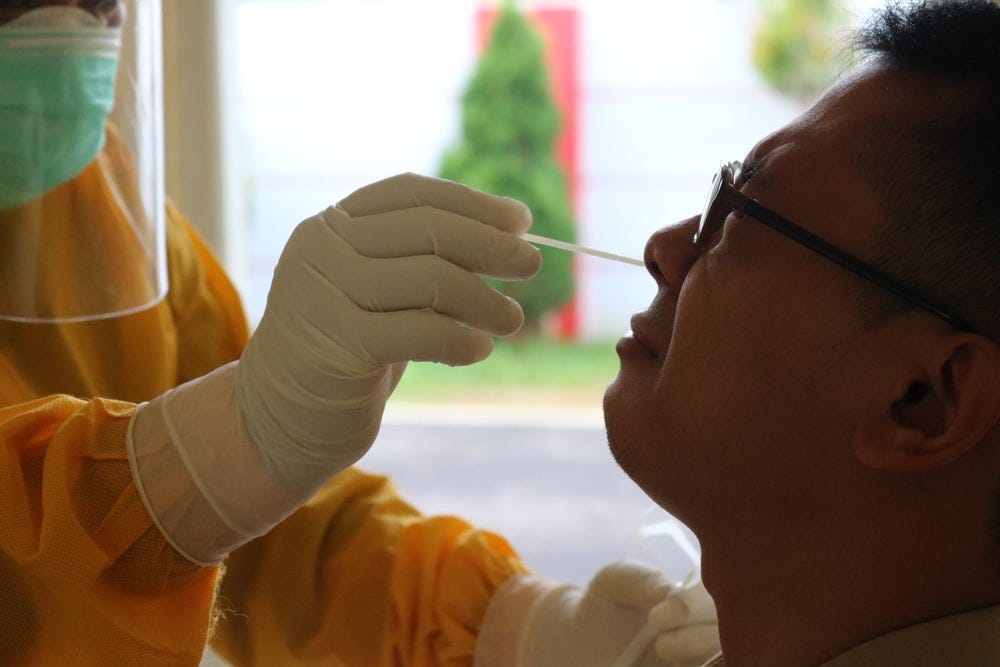
No amount of effort to make the approval process more efficient can overcome a society whose actions are currently straining public services like COVID testing and hospital care. Canadians have made the choice to roll the dice with COVID.
Who, I wonder, sitting on the Opposition benches would Fraser Macdonald recommend be given the authority to override Health Canada's procedures, assessments, and determinations, and make the "reasonable risk assessment" regarding Canadians' health and safety? Michelle Rempel Garner, Pierre Poilievre, and Erin O'Toole have been harsh and loud critics of Health Canada's (and Prime Minister Trudeau's) approach to ensuring the safety and efficacy of COVID-19 drugs, tests, and treatments. Should Rempel Garner, Poilievre, and O'Toole be tasked with overriding Health Canada when they see fit, and assess the risk to Canadian's health? If not them, if not Health Canada, if not the government, then who?